The birth equation of Quantum Mechanics
Quantum theory, the branch of physics based on quantization, began in 1900 when Max Planck published his theory explaining the emission spectrum of black bodies. In that paper Planck used the Natural system of units invented by him the previous year.
The quantum black-body radiation formula, being the very first piece of quantum mechanics, appeared Sunday evening October 7, 1900, in a so-called back-of-the-envelope calculation by Planck. It was based on a report by Rubens (visiting with his wife) of the very latest experimental findings in the infrared. Later that evening, Planck sent the formula on a postcard, which Rubens had the following morning. A couple of days later, he could tell Planck that it worked perfectly. As it does to this day. The following is the equation that started quantum mechanics.
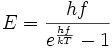
The above formula is the basis of quantum mechanics. In this specific equation, there are two parameters written in the present form by the symbols used today: h is the new Planck's constant, and k is Boltzmann's constant. Both have now become fundamental in physics, but that was by no means the case at the time. The "elementary quantum of energy" is hf. But such a unit does not normally exist, and is not required for quantization.
From the experiments, Planck deduced the numerical values of h and k. Thus he could report, in the German Physical Society meeting on December 14, 1900, where quantization (of energy) was revealed for the first time, values of the Avogadro-Loschmidt number, the number of real molecules in a mole, and the unit of electrical charge, which were more accurate than those known until then. This event has been referred to as "the birthday of quantum mechanics".